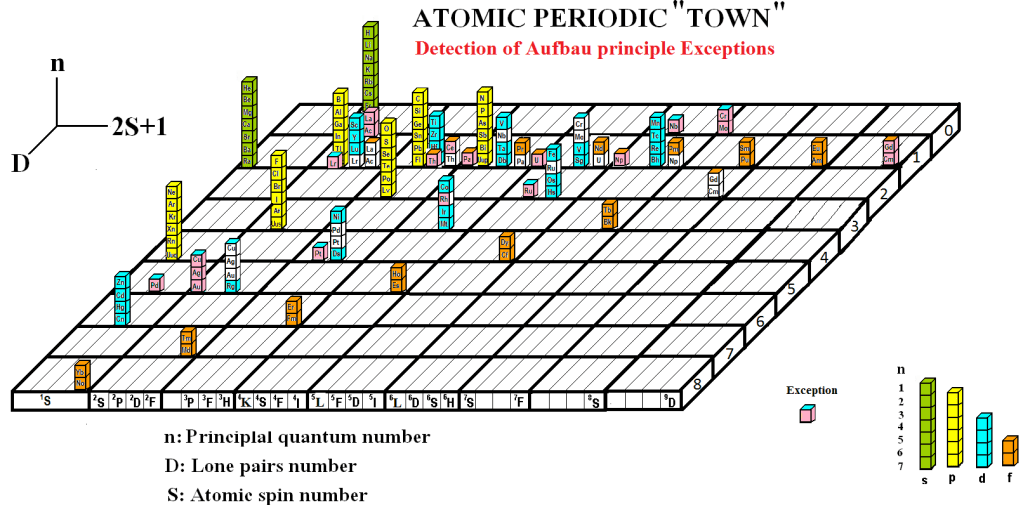
The exceptions are characterized by electronic configurations in which unpaired electrons occupy different subshells. In the common situation where these elements change valence, the number of electron pairs differs from that predicted by the standard periodic table, causing them to shift to new positions. Even when the valence remains the same, the type of orbitals involved may change, which also results in a repositioning within the table.
The figure below illustrates the periodic table with the revised positions of these exceptions.
Laisser un commentaire