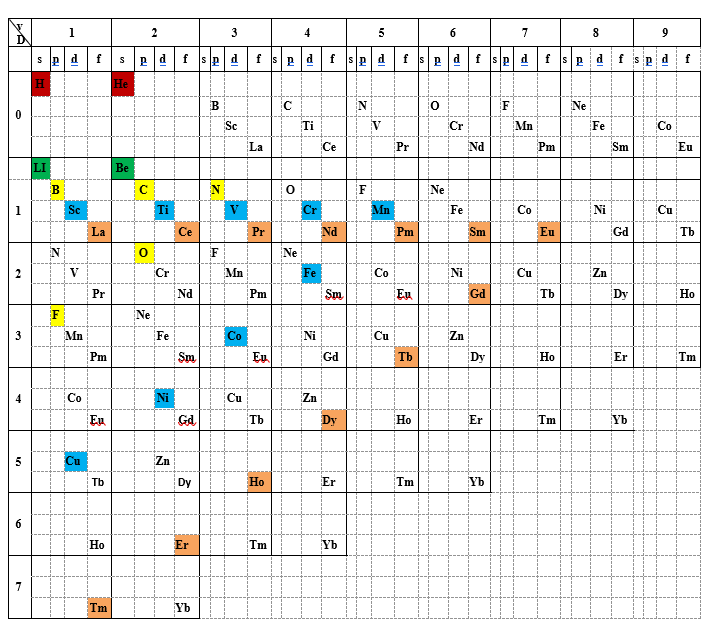
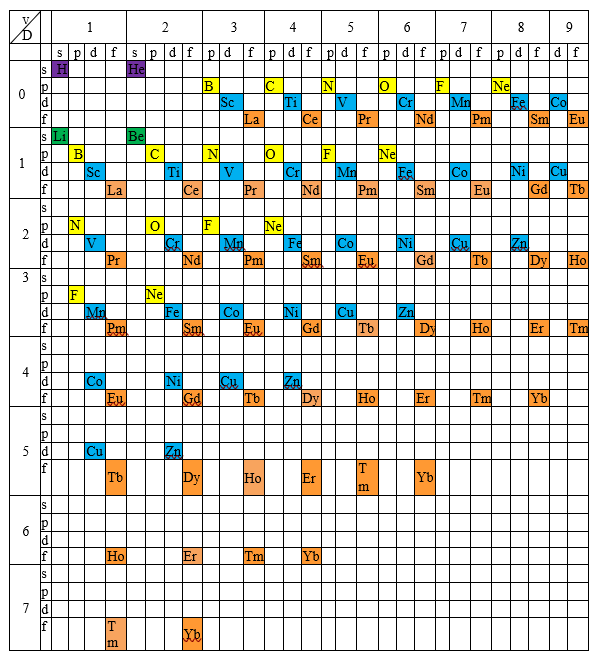
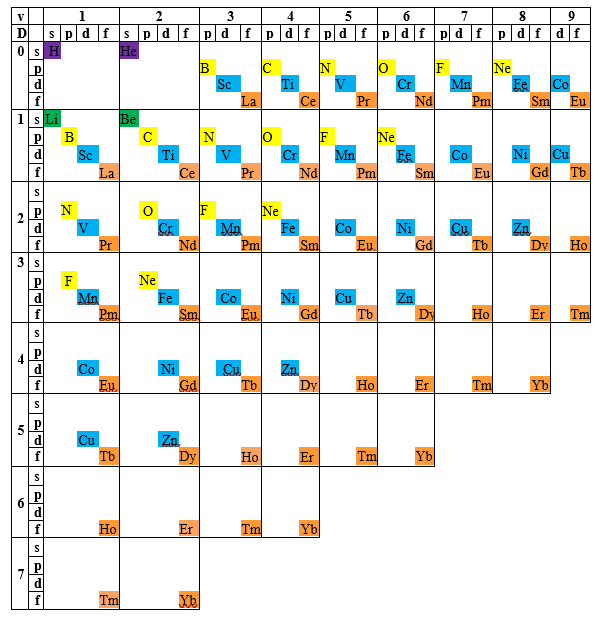
The fact that exceptions occupy different positions in the periodic table suggests the possibility of filling the empty or partially filled squares by placing atoms within molecules according to their electronic configurations. In essence, this approach makes use of their valence. The result is a new arrangement, which we call the generalized periodic table. Examples illustrating its usefulness will be provided later in this post. We limited the variation of valence to 9, since this value represents the highest known valence to date, observed in the molecular anion ReH₉²⁻.
Laisser un commentaire